Molecular Testing for Thyroid Cancer: What to Know

Introduction
Thyroid nodules are very common. Thyroid nodules are growths that occur in the thyroid gland forming a lump or bump within the otherwise smooth thyroid surface. Thyroid nodules occur in 40% of women and 30% of men during their lifetime, and possibly more people are affected than diagnosed. Many people are unaware they have thyroid nodules or even thyroid cancer. When people hear they have a thyroid nodule they worry about cancer and the need for surgical removal. Thyroid cancer only accounts for approximately 5-15% of all thyroid nodules as best we know.
Molecular Testing for Thyroid Cancer: What to Know
The diagnosis of thyroid cancer continues to evolve and improve thanks to better imaging (high-resolution ultrasound primarily), pathology/cytology, and more recently, molecular testing for thyroid cancer. When a needle biopsy or fine needle aspiration (FNA) is done to diagnose a thyroid nodule, there is not always a definitive answer (benign or cancerous). For these indeterminate nodules, there are now further molecular/genetic testing options to obtain more information about the diagnosis. Below I will discuss what you need to know about molecular testing for thyroid cancer.

Molecular testing for thyroid cancer starts with ultrasound and needle biopsy
An excellent, high-resolution thyroid ultrasound is the cornerstone of determining whether a thyroid nodule is malignant (cancerous) or benign (non-cancerous). Blood tests almost never diagnose thyroid cancer. A fine- needle aspiration (FNA) biopsy is frequently needed to obtain more information about how a thyroid nodule is behaving. We just call this a “needle biopsy”. A needle biopsy is typically done for larger nodules (>1 cm or 3/8 inch), symptomatic nodules, nodules that have suspicious features on ultrasound, nodules that are changing in appearance, and/or thyroid nodules that have increased in size/volume. The results of a thyroid needle biopsy are categorized according to the Bethesda System for Reporting Thyroid Cytopathology for Thyroid Nodules. The diagnostic categories are as follows:
1- Nondiagnostic or unsatisfactory
2- Benign
3- Atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS)
4- Follicular neoplasm or suspicious for a follicular neoplasm
5- Suspicious for malignancy
6- Malignant
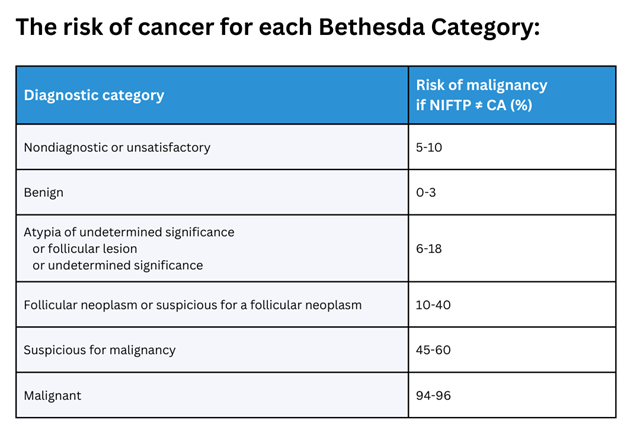
Note: Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) is a non-malignant tumor that is classified in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) as a borderline or pre-malignant lesion. Since 2016, NIFTP has been classified in the TBSRTC as a noninvasive encapsulated follicular variant papillary thyroid carcinoma (eFV-PTC) with indolent or benign behavior and is no longer considered carcinoma.
As you can see, Bethesda Categories 3-5 do not yield a definitive diagnosis (often called indeterminate). As such, a few companies have developed further molecular testing for thyroid cancer.
Molecular testing for thyroid cancer: The basics
Over the past 15 years or so, there are companies (Afirma, ThyroSeq, ThyGeNEXT/ThyraMIR, etc.) that have developed further testing for FNA biopsy samples that are indeterminate. These molecular/genetic tests are most often done on Bethesa III and Bethesda IV nodules/results. Sometimes they are done for Bethesda V nodules, but with those being very suspicious based on cytology (how the cells look under a microscope), most often thyroid surgery is done without any further testing or molecular analysis.
These molecular tests for thyroid cancer look for different markers/changes/mutations in the cells to determine if cancer is present, absent, or more likely/less likely present. If the results are not definitive, these molecular/genetic tests will give a percentage risk or chance that thyroid cancer is present (50%, 75%, 95% etc.). Very frequently, these tests are helpful in determining how best to move forward with monitoring or surveillance of the nodule, surgery, and the extent of surgery (thyroid lobectomy vs. total thyroidectomy).
Molecular testing for thyroid cancer: The implications
Simply put, molecular testing for thyroid cancer helps guide clinical decision- making. As noted above, when the needle biopsy yields an atypical or suspicious result (Bethesda III or IV), these molecular/genetic tests can help the patient and clinician determine whether thyroid surgery is needed. The results of the test can yield a risk of cancer ranging from 3-4% or less up to 99% depending on the molecular/genetic profile of the cells from the needle biopsy. For nodules that come back with a 90- 99% chance of cancer, expert thyroid evaluation and surgery is required. Nodules that return benign results on the molecular test (3-4% chance or less of cancer) can be monitored with regular annual ultrasound assuming the patient and doctor are comfortable with that option.
While molecular testing for thyroid cancer continues to improve, the results should not be evaluated in a vacuum disregarding other clinical features and symptoms (i.e. size of nodule, concurrent thyroid disease, symptoms from the nodule, etc.). Like anything else in medicine, the molecular/genetic tests are not perfect either. Our surgeons have removed many nodules that were benign on molecular testing but turned out to be thyroid cancer. Additionally, there are a couple thyroid cancers that are almost impossible to diagnose with the molecular testing. In particular Hurthle Cell/Oncocytic cancer is almost never diagnosed on molecular testing. In conclusion, while the molecular tests for thyroid cancer are of great utility, they are not perfect and should be one part of the whole clinical picture for thyroid nodule evaluation and treatment.
Summary
Thyroid nodules are very common as noted above. Work-up and diagnosis of thyroid nodules begins with an expert high-resolution ultrasound and needle biopsy (FNA) if needed. When the biopsy result is indeterminate, often times molecular/genetic testing on the FNA sample will give more information regarding whether the nodule is cancerous or benign. The molecular testing can sometimes help determine the extent of surgery needed based on certain markers or mutations noted that yield information on if the nodule is a more aggressive cancer. While these molecular tests for thyroid cancer are very good and continue to improve, they are not perfect. There are still some rare thyroid cancers (i.e. Hurthle Cell/Oncocytic thyroid cancer) that are almost never diagnosed on these tests. As such, the results should be taken in totality with the other clinical information.
Our team of thyroid experts is here to help and guide you every step along the way during the diagnosis and surgery for your thyroid disease. The Clayman Thyroid Center is dedicated to providing world-class care for thyroid cancer and thyroid disease. To learn more and become a patient, please see our resources below.
Additional Resources
- Become our patient by filling out the form at this link.
- Learn more about The Clayman Thyroid Center here.
- Learn more about our sister surgeons at the Scarless Thyroid Surgery Center, Norman Parathyroid Center, and Carling Adrenal Center
- Learn more about the Hospital for Endocrine Surgery.